Recent Highlights
INSECT DOCTORS PhD graduate wins Dutch Entomology PhD thesis prize 2024
By Monique van Oers, December 27, 2024

Luis Hernández Pelegrín has been awarded the Dissertation Prize for the best thesis in entomology in the Netherlands of the past year. This annual prize, presented by the Netherlands Entomological Society (NEV), recognises PhD graduates who have conducted groundbreaking research in the field of entomology.
Luis PhD thesis deals with the Mediterranean fruit fly (Ceratitis capitata), a pest species that causes significant agricultural damage worldwide, particularly in citrus production.
His work identified the presence of as many as 13 different viruses in this species. Additionally, he explored how these viruses are transmitted and their effects on the fly’s development. Follow this link for the original version of this news item.,
A week in Vienna
By Monique van Oers, August 23, 2024
INSECT DOCTORS was well represented and highly visible at the Annual Meeting of the Society for Invertebrate Pathology (SIP), held from July-28- August 1, 2024 at the Technical University in Vienna, Austria.
The meeting was organised by Adly Abd Alla (partner FAO/IAEA) and Joerg Wennmann and Johannes Jehle (both from beneficiary JKI). A number of INSECT DOCTORS PhD candidates/recent graduates contributed scientifically to the meeting with high quality oral presentations: Luis Hernández Pelegrin, Jirka Manuel Petersen, Edouard Bessette, Lim Fang Shiang, Sam Edwards and Hannah-Isadora Huditz. Their contributions were well received:


- Luis was awarded the Mauro Martignoni Award, the highest student award offered by the SIP, for his contribution “Effect of domestication on the repertoire of viruses and bacteria associated with the Mediterranean fruit fly, Ceratitis capitata”
- Jirka won the first prize in the virus division for an oral presentation entitled: The “nudist” viruses from prehistoric times: Data-driven discovery of novel nudiviruses from ectoparasitic insects prompts a taxonomic and evolutionary re-evaluation within the family Nudiviridae”
- Edouard and Jirka gave excellent invited symposium talks in the Microsporidia and Division for Beneficial Invertebrates (DBI), respectively.
- Sam received a travel grant from the Microsporidia Division for a paper co-authored with Edouard; Jirka received a travel grant from the Virus Division and Hannah from DBI.
- Lim was one of the conference helpers at the meeting, and Hannah assisted in an earlier phase.
Elisabeth Herniou (Beneficiary CNRS) gave an excellent overview of many of the INSECT DOCTORs results in her symposium presentation “Challenges of pathogens detection in insect mass rearing: Advances from the Insect Doctors program for improved diagnostics” in the Pan-Division Symposium between Viruses, DBI & Bacteria Divisions, organised by Christina Nielsen-LeRoux, Helen Hesketh & Vera Ros (Beneficiaries INRAe, UKCEH and WU). The course outcomes of the INSECT DOCTORS course on mixed stressor-modelling for mass-reared insects were presented by Ellie Dearlove (course teacher, ADAS), in a talk co-authored by all course participants, as well as Helen Hesketh (Beneficiary UKCEH), the course organiser.

And as an extra bonus: Sam won the 1st prize in the 5k run competition in the category “men under 40”, Eduard the 2nd and Jirka the 3rdprize,
whilst Vera Ros and Helen Hesketh picked up 2nd and 3rd places in the category female walkers
Well done everyone!
From the laboratory to the fashion day
By Hannah-Isadora Huditz. First Published November 18, 2023

My name is Hannah-Isadora and I am ERS7 working on virus infection in tsetse flies. I wrote the first news report in May 2021. Ever since a lot of interesting and innovative articles have been published on this website and I am very glad to share my research with you again. During the last year I had the chance to learn from various researchers in different laboratories. I spent six months at the Wageningen University followed by six months at the Exeter University in Cornwall. In both locations, I was able to improve my cell culture techniques and got encouraged to try new experimental techniques. This brought me to my current project: Separation and isolation of a tsetse Ifla-and negevirus. My PhD objective is to find out what the impact of these viruses is on the health and fitness of the tsetse fly. To understand the viruses’ role, I would like to study their mode of action independently. We used two different approaches: One consists of isolating the virus by passaging via different insect cell cultures and the other by creating infectious clones. We were able to find out that Spodoptera frugiperda Sf9 cells and Aedes albopictus C6/36 cells could only maintain the Glossina morsitans moristans iflavirus infection, but not a Glossina morsitans morsitans Negevirus infection. This will allow us to isolate the iflavirus via ultracentrifugation from the culture fluid. Our goal is now to understand the impact the iflavirus has on the tsetse fly. This will provide us useful information for the mass raring of the tsetse fly which is necessary for the application of the sterile insect technique.
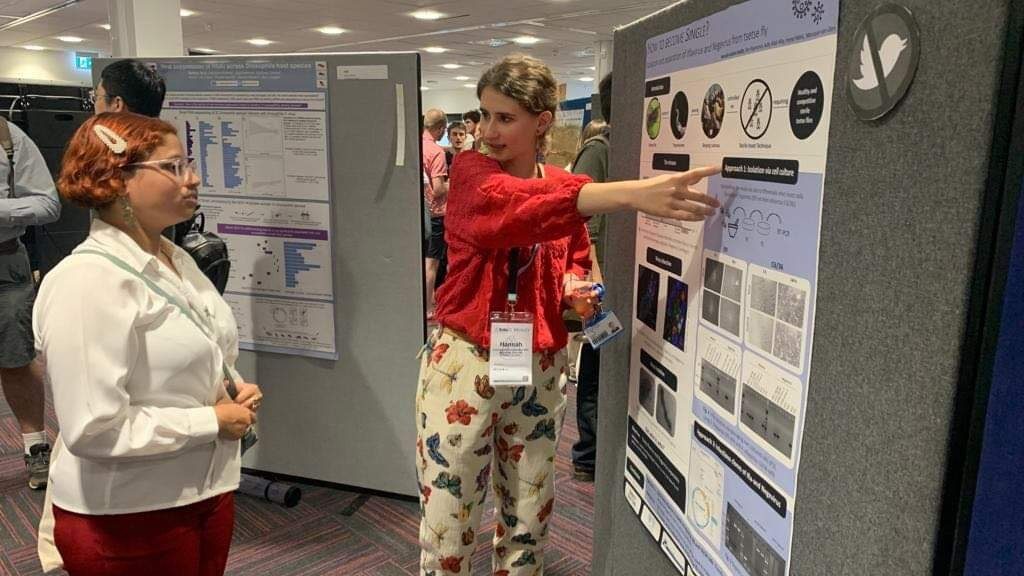
I had the opportunity to present these results at two conferences. At the International Congress of Invertebrate Pathology and Microbial Control (SIP2023) in Maryland, USA and at the Conference of the Royal Entomological Society, in Cornwall UK. In the latter, I shared my findings via a scientific poster. During the second conference we had an entomological fashion day. At this event the participant with the best entomological outfit was selected. It was stunning to see everyone coming up with such nice ideas, from bee inspired earrings to beautiful butterfly skirts. I could convince the jury with insect pans, a fitting bag and the must have for each entomologist: a bucket hat. I received the first price, which was a surprise to me, due to the other very strong participants.
I am now looking forward to my last year of my PhD where I will study the impact of the ifla- and negevirus on the fly’s immune system. I am curious on what I will find and which new challenges will come along with it.
Attending the Society for Invertebrate Pathology (SIP) Meeting 2023
By Jozsef Takacs
First Published September 12, 2023
For years during COVID, many meetings were held online and this was no different for the Annual Meeting of the Society for Invertebrate Pathology (SIP). It was thus with great pleasure that, this meeting was held in-person, in the College Park of Maryland, USA, from July 30th to August 3rd, 2023, and its participants could share research, new ideas and create new connections again for the first time since 2019.

The society dates back to 1967 and we could see it as the alma mater of the Insect Doctors program, as many of the work package leaders, supervisors and promotors knew each other via the annual SIP meetings. Being able to see the persons whose work and publications were inspiring to us as young scientists was a nice opportunity.
Eight PhD students from the Insect Doctors program gave presentations for the SIP Divisions of virus, bacteria, microsporidia, fungi and beneficial invertebrates. Beside, presenting in their relevant divisions, several of the students also received travel grants to ease on their costs of travelling outside the old continent. Coverage of almost every division of the SIP by the Insect Doctors’ PhDs nicely shows the complexity of the program and how it aims to cover every possible aspect relevant for the diseases in mass reared insects. The PhDs of the Insect doctors program received valuable feedback and seized the opportunity to engage in inspiring conversation about their work with fellow scientist. The meeting was visited by scientists from Europe, North America, South America, Asia and Australia. To have such a diverse scientific community is of high value, as different expertise and backgrounds are being put together for the best possible outcome.
Beside the scientific part, the program also gave opportunity to explore the well-known landmarks of Washington DC, such as the Whitehouse, the Capitol and the National Mall. Combined with the traditional 5K run of the SIP, the program was full of science, culture, sports and human interaction.
Viral journey unfolded in buzzing world of Black Soldier Flies (BSF)
By Robert D. Pienaar
First Published September 1, 2023
Keeping things on the fly, the focus on BSF infecting pathogens has expanded rapidly. Once thought to be invincible against pathogens, BSF are revealing their vulnerabilities, prompting a deeper dive into their health and rearing practices. So…what is the next step?

Research on BSF and pathogens has begun to kick-off over the last 3 years. Multiple publications have shown the potential of some bacteria and fungi to cause signs and symptoms of disease in BSF under certain laboratory conditions. In line with this, this year it has come to light that one of the problems observed in industrial BSF rearing can be caused by a bacteria, Paenibacillus thiaminolyticus.
Adding a twist to the tale, in the last year two separate studies, one by the Insect Doctors program and another by US researchers have spotlighted viruses linked to BSF. While we only introduced one of the virus candidates that we were characterizing at the time, together, the list of viruses associated with BSF now stands at eight. In addition, viral-like elements (“virus fossils”) found in BSF genomes provided evidence of four other unknown viruses having historical interactions with, BSF.
Embarking on this scientific voyage from scratch was indeed challenging. From exploring and compiling bioinformatic virus discovery pipelines for non-model organisms, to finding general and relatively easy to use laboratory-based virus detection/discovery methods. For six of the viruses, we have been designing rapid screening tests which have already been used to screen for these viruses in colonies from multiple BSF farms. There is now an extended list of farms from across the globe who are requesting access to testing. Suggesting that any company experiencing entomopathogen-related issues in BSF rearing, is likely, not alone. In light of this, we were incredibly fortunate that people were willing to share data with us and companies willing to give us a chance to be privy to their operations, experiences, and samples.
In BSF obtained from multiple sources, some tested positive for either of six BSF virus candidates. From these BSF, we have managed to successfully isolate one of the virus candidates, from symptomatic BSF. Unfortunately, since there were no viral bioassays for BSF before, thus to determine infectivity of this virus, we had to be a little creative. It took some time, but we managed to develop and optimize bioassays for BSF adults, larvae and pupae which are appropriate for determining infectivity and transmission of viruses in BSF. Another issue is that not all pathogenic viruses will consistently cause disease in their host, however, we now have tools to simulate Koch’s postulates for viruses in BSF, previously a non-model organism.
While more scientific publications are trying to shed some light on BSF pathogens and pathology, few cater to non-expert audiences. Part of the Insect Doctors commitment is to produce open science and hopefully what we achieve by the end of the programme will help further promote collaborations between researchers and insect mass-rearing counterparts.
The home stretch
By Loretta Mugo-Kamiri
First Published August 22, 2023
It is incredible how three years have gone by so fast. As I draw closer to the end, I reflect with gratitude on all the lessons, connections and experiences that have come about during my Ph.D. journey.

I am ESR 14 and I continue to work on the relationship between diet-gut microbiome and pathogens in Lepidoptera species. Broad questions arise about the role of the gut microbiome in insects. Numerous publications have reported their functions in digestion, growth and development, metabolism, nutritional supplementation as well as immune protection. For the past year, I have been busy trying to understand whether the gut microbiome provides protection against pathogens or vice versa. During this period, I have spent time in the lab carrying out large bioassays with a variety of pathogens such as baculoviruses and entomopathogenic bacteria such as Serratia marcescens and Pseudomonas entomophila. In addition, it has been an exciting time learning new ‘omics approaches such as Dual-RNA sequencing and transcriptomic data analysis. We have obtained impressive data pointing towards the protective role of the gut microbiome against pathogens; however, this is highly dependent on host species, the pathogen and the composition of the gut microbiome.
This summer, I have had the privilege to present my work in two fantastic conferences. One of them was the CNRS-Jacques Monod conference on insects as models for infection biology held in Roscoff, France. This conference stood out for me because some of the presentations made included decades worth of work from leading international scientists and this made me appreciate the time and dedication put in to answer some of science’s most pressing questions. I also just recently attended the Society for Invertebrate Pathology (SIP) conference held in College Park, Maryland where I received an award for the best oral presentation for my talk titled “Diet dependent virulence effects of gut symbionts: The symbiosis of Enterobacter cloacae and Plutella xylostella”. In both of these conferences, I had great opportunities to network with other scientists and make what I believe will be long-term connections.
On this home stretch, I focus on finishing the little that is left of lab work, writing up my thesis and finally becoming an insectdoctor, before the next exciting adventures in science.
For more updates on my work, find me on LinkedIn and twitter (now X) @LorettaMugo
Interactions between insects and pathogenic fungi – Tenebrio Molitor
By Anna Slowik MSc.
First Published May 30, 2023
Exploring the fascinating world of insect-pathogen interactions: My experience working with yellow mealworms at Leeds Uni (UK).

Working with insects may not be for everyone, but for me, it is an exciting and engaging experience. For the final year of my program, I moved to Leeds to work under the supervision of Steve Sait at the University of Leeds School of Biology, where I am finishing the last of my experimental work.
For the final aspect of my PhD project, I’ve focused on understanding the interactions between insects and pathogenic fungi. Specifically, I’m interested in how the course of infection is influenced by environmental factors, like temperature. To study this, I’m using the model organism Tenebrio molitor, commonly known as the yellow mealworm. Working with mealworms has been a fascinating experience. These insects have a rather long lifecycle of three months but reproduce quickly and gregariously. They are also simple to maintain and require little space, which is especially important in a busy lab filled with other active experiments. Also, quite importantly, they do not fly around or escape easily. These characteristics make yellow mealworms idea model organisms for studying various biological processes, like host-pathogen relationships.

To investigate how temperature mediates disease outcomes in host-pathogen relationships, I measured the growth of both the fungal pathogens I work with (Metarhizium spp) and the mealworms separately at different temperatures, as well as how the infection progressed. I chose four temperatures, including the optimal temperature for growth for the fungi, the optimal temperature for the insects, and then two more temperatures that would be sub-optimally high and low for both organisms. The work has just finished, so I am excited to analyse the data and see what the outcome is. I hope my results will show how well the fungi and insects grow individually at a range of temperatures, and then how the infection progresses at these temperatures. This provides information on which are the more important factors for the infection processes – the thermal needs of the fungi or those of the insects. These findings could have important implications for understanding the dynamics of insect-pathogen interactions in natural and agricultural systems.
Perhaps even more exciting than the findings themselves has been the process of conducting this research. I appreciated getting to know the intricate lives of the mealworms and learning more about their biology and behavior. I have been amazed by the complexity of the interactions between these insects and their environment, and the many ways in which subtle changes can have profound effects on their health and well-being. Overall, my experience working with insects for my final experiment has been incredibly rewarding, and I am excited to continue exploring these fascinating creatures and the ways in which they interact with the world around them.
Bugs in Bugs: The Role of Probiotics and Prebiotics in Maintenance of Health in Mass-Reared Insects
By Carlotta Savio
First Published May 16, 2023
I’m Carlotta Savio, ESR 13 in the Insect Doctor Program focusing on the effects of probiotic and diet composition on Tenebrio molitor performances in presence of entomopathogens. The research project is mainly carried out at INRAe (FR) and Wageningen University (NL) with the involvement of the University of Copenhagen (DK).
First reflections on probiotics applications in mass reared insects ended in the publication of the review paper “Bugs in Bugs: The Role of Probiotics and Prebiotics in Maintenance of Health in Mass-Reared Insects” https://doi.org/10.3390/insects13040376

In the meantime, potential probiotic bacterial strains have been tested on Tenebrio molitor for assessing their effects on insect performance, immune responses and microbial community composition in optimal conditions andin presence of entomopathogens. Questions related to the effects of probiotics provision and feed on insect chemical composition have been explored during the last research period spent in between the Laboratory of Entomology and the Food Quality Department at Wageningen University (NL). Currently, I am writing down the output of the research and I am finding fascinating the importance of the role of the microbiota, especially mutualistic symbionts, on host health maintenance and food and feed quality.

If you want to know more about yellow mealworms symbionts.. with the review “Harmful and beneficial symbionts of Tenebrio molitor and their implications for disease management” we offer an overview on its symbionts, detection methods and possible strategies for preventing diseases in mass rearing systems.
Taking part of this program, discussing, learning and collaborating with the other “going to be” Insect Doctors is being a unique, inspiring and precious chance.
Follow me on twitter @carlottasavio1 for staying updated on my activities.
Overcoming obstacles and expanding my horizons: Highlights of my PhD journey
By Pascal Herren
First Published May 9, 2023
A lot has happened since my last update in January 2022. I completed my practical work at the UKCEH (UK Centre for Ecology & Hydrology, Oxfordshire), moved to a new city, visited a mealworm producer, and last but not least I attended several conferences and courses which allowed me to travel through Europe. Here are some of the highlights of my PhD journey so far.
One of the questions I would like to answer in my work is how carbon dioxide (CO2) affects insect-pathogen interactions. The major challenge I encountered during this study was setting up an experimental system that would allow me to perform my experiments. Initially, we planned to use climate-controlled rooms for the experiments, but we soon realised that this was not feasible due to low CO2 concentrations and fluctuating relative humidity, which would have introduced another uncontrollable stressor to my experiments. We then decided to use smaller incubators in which the CO2 concentrations could be elevated to the desired levels. However, this presented a new obstacle because the insects themselves generated enough CO2 to increase the concentrations inside the incubators. To overcome this, I designed a ventilation system that maintained stable CO2 levels inside the incubators. Fortunately, despite my lack of engineering background, I was able to overcome these challenges and conduct all the planned experiments successfully.

Aside from conducting extensive experimental work, I had the opportunity to travel to various locations for other opportunities. One highlight was visiting ‘Mr. Bug’ (https://www.mrbug.co.uk/) together with my supervisor Helen Hesketh. This company specialises in producing yellow mealworm protein-based dog treats for the pet food industry. During our visit, we had the pleasure of touring their facility and engaging in stimulating discussions with their team. It was very motivating to see that my experiments and findings are relevant to the insect production industry and for the producers to explain the practicalities and challenges of rearing insects.
Later in February, I had to say goodbye to my dear friends and colleagues at UKCEH and I packed my belongings to move to the third and last station of my PhD journey, which is Leeds. At the University of Leeds, I received a warm welcome from my supervisor Alison M. Dunn and the lovely people in her group, who helped me to settle in quickly. In Leeds, I am focusing on the data analysis of my last two chapters and writing up my thesis. However, I have also lined up some welcome distractions from writing, such as INSECT DOCTORS courses in France in May, a visit to the company YNSECT, and the participation in the annual conference of the Society of Invertebrate Pathology in the United States in August. Even though, there is still a lot of work to complete before submitting my thesis, I am excited and motivated to tackle this final stage of my PhD.
PhD time flies… but also mosquitoes! An update
By Alessandro Roman
First Published March 21, 2023
I am Alessandro Roman, Insect Doctors’ Early Stage Researcher 15. My PhD project is part of Work Package 3, with the aim to gain knowledge on how to increase fitness of mass reared insects. Since January 2022, I am based at Wageningen University and Research, precisely working in the Plant Science Group – Laboratory of Entomology… and yes… as you can see from my previous Research Highlight (https://insectdoctors.eu/en/insectdoctors/version-of-impact-of-bacterial-symbionts-on-host-growth-and-mating-competitiveness-in-mass-reared-mosquitoes.htm), I am still exploring one of the most intriguing insects on earth, mosquitoes.

My journey started in the United Kingdom, at the University of Exeter, based in Penryn, a fascinating town surrounded by Cornish landscapes. Here, I established an Aedes aegypti rearing, also known as the yellow fever mosquito, by reading and spending days setting the best conditions and practicing how to handle the necessary equipment for supplying blood. This mosquito is considered the main vector of several arboviruses that are responsible for numerous human diseases 1. The risk of vector-borne diseases will increase due to the important role of climate change 2,3, exacerbating the risks related to disease burden and health inequity, and lead to slowing down socioeconomic development and strain health services 4. To effectively control mosquito populations, mass release techniques are gaining interest, and hence the fitness of mass reared insects.

Bacterial symbionts can play an essential role in individual mosquito development and fitness. Our experiments were performed with the aim to understand how development and time to pupation (two important fitness parameters for mass rearing production) were affected by the presence of specific bacterial symbiont species in the mosquito gut. Our interesting results led to new questions related to the mosquito microbiota, and its dynamic. Thanks to this experience, I had the opportunity to gain and improve my skills in bioinformatics, specifically related to analysis of Illumina sequencing data.
Subsequently, the time to join Wageningen University arrived. It was challenging to move to a different lab, in a different country, and adapt in a short period of time, in order to have myself running again in this stimulating environment. My interest in the research and the passion of the One Health Entomology Group in Wageningen helped me getting started in the lab. Seeing the first results obtained in the United Kingdom, and the opportunity offered by the laboratory of Entomology, we decided to explore how different mosquito species and strains are influenced by the selected symbionts, concerning fitness parameters, and not only…
Interesting results will come soon… stay tuned!
The travels for my PhD journey did not end yet, I will fly to Austria in few months, with the destination of the Joint FAO/IAEA Division of Nuclear Techniques in Food and Agriculture.
References:
- Espinal, M. A. et al. Emerging and Reemerging Aedes-Transmitted Arbovirus Infections in the Region of the Americas: Implications for Health Policy. Am. J. Public Health 109, 387-392, doi:10.2105/ajph.2018.304849 (2019).
- Iwamura, T., Guzman-Holst, A. & Murray, K. A. Accelerating invasion potential of disease vector Aedes aegypti under climate change. Nature Communications 11, 10, doi:10.1038/s41467-020-16010-4 (2020).
- Rocklöv, J. & Dubrow, R. Climate change: an enduring challenge for vector-borne disease prevention and control. Nature Immunology 21, 479-483, doi:10.1038/s41590-020-0648-y (2020).
- Messina, J. P. et al. Mapping global environmental suitability for Zika virus. elife 5, e15272 (2016).
