WP1 Research Projects:
Pathogen-host interactions
Discovering interactions between pathogens and insect hosts,
and determining abiotic and biotic triggers for disease outbreaks.
Project 1
Nudivirus replication mechanism
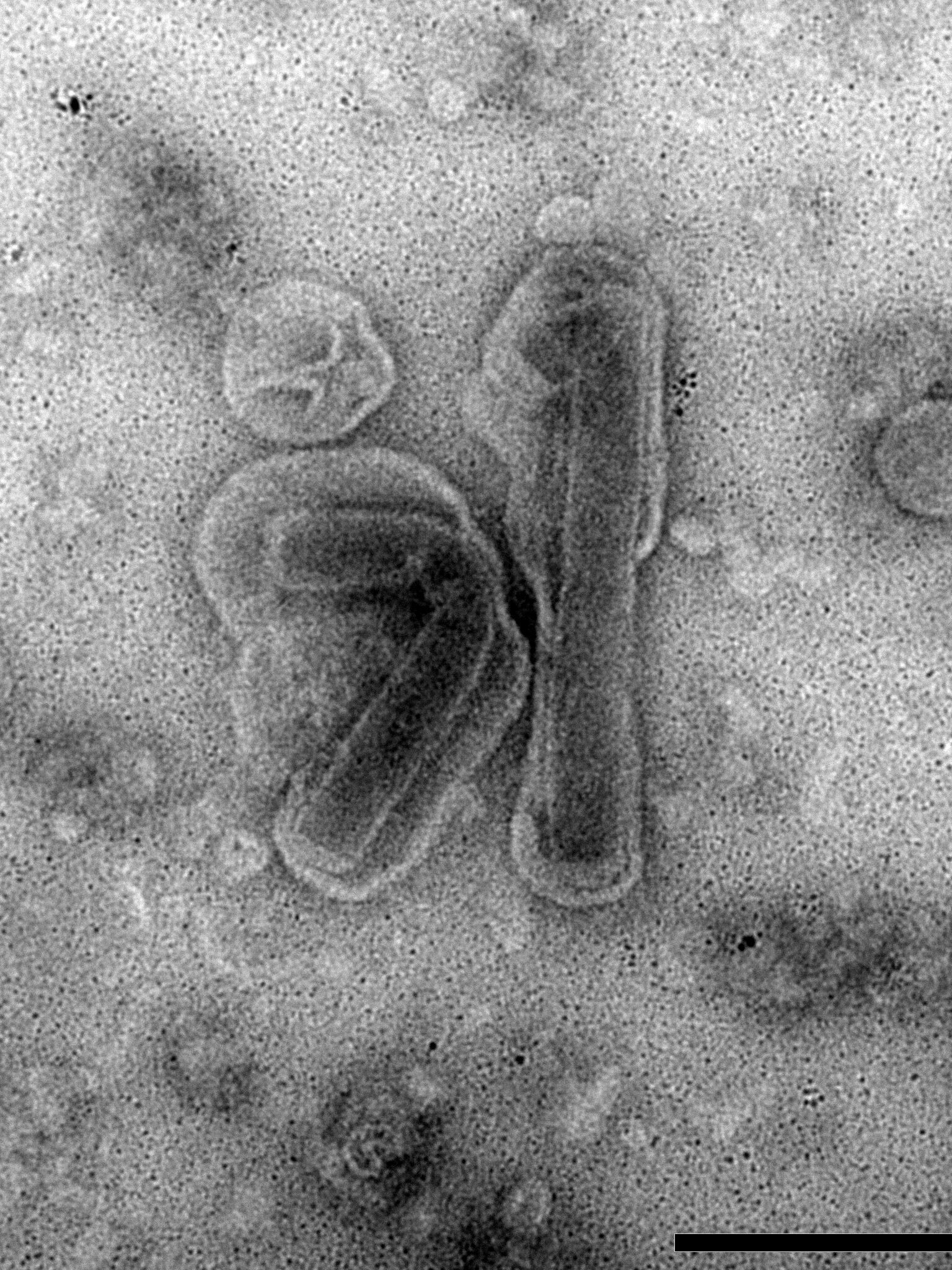
The research project “Nudivirus replication mechanisms” aims to unravel the replication mechanisms and gene functions of nudiviruses (Nudiviridae). These invertebrate-infecting viruses are phylogenetically related to endogenous bracoviruses (Polydnaviridae) found in parasitoid wasps. The Heliothis zea nudivirus 1 (HzNV-1) genome was found in its host as a persistent infection. Experiments with nudiviruses and bracoviruses aim to reveal whether these viruses have similar replication processes.

PhD candidate
Jirka Manuel Petersen
Supervisors
- Monique van Oers, Wageningen University (NL), Laboratory of Virology
- Jean-Michel Drezen, University of Tours (FR) and IRBI?CNRS
(Jirka M. Petersen)
Outline
One of the most diverse virus families found in insects are nudiviruses (from Latin, nudi = naked or uncovered) of the family Nudiviridae. They have been found to infect different insect orders, including crickets, beetles, moths and flies, but also marine invertebrates, such as lobsters and shrimp. Nudivirus infections in invertebrates can reduce their hosts’ fitness and under certain conditions lead to their death. Therefore, it is important to study the impact of these diverse viruses to prevent devastating losses in aquacultures and the uprising industry of insect rearing. Conversely, the harmfulness of a beetle-infecting nudivirus (Oryctes rhinoceros nudivirus, OrNV) has been used to control infestations of this insect pest in palm tree cultures in parts of Oceania, providing another relevant reason to study this virus family.
The species Heliothis zea nudivirus comprises two strains, HzNV-1 and HzNV-2. Its name originates from its natural host Helicoverpa (formerly Heliothis) zea, commonly known as the corn earworm. Caterpillars of this moth pose a threat to corn and other crop plants. HzNV-2 is sexually transmitted between individuals or to the offspring via infected ovaries, and causes disease in larvae and adults of H. zea. HzNV-1, on the other hand, was isolated as a persistent viral infection in a cell line derived from H. zea ovarian tissue and was not capable of infecting actual insects. HzNV-1 can induce latent and lytic infections in different insect cell lines and may persist in the host cells through viral genome integration.
It was shown that the integration of an ancestral nudivirus into the genome of an ancestor braconid wasp 100 million years ago gave rise to the clade of endogenous bracoviruses. Therefore, it is reasonably assumed that the replication mechanisms and machinery of bracoviruses derive from its nudiviral ancestor. However, little is known how bracovirus replication is maintained after viral genome integration and what genes facilitate crucial functions in this state. Our main research aims to reveal similarities in the gene functions of nudiviral and bracoviral replication by conducting comparative studies with the latest molecular, genomic analytical tools and microscopic techniques.
Project 2
Behavioural manipulation and co-evolutionary processes between fungi and flies
The expanding industry of insect mass rearing aims to produce millions of insects that provide beneficial services to humankind, such as biocontrol agents, animal feed and human food. However, this intensive production system also incurs potential insect health consequences from rearing at such high densities. This developing industry is already facing disease outbreaks in their insect cultures from insect-pathogenic bacteria, viruses, fungi, and other pathogens.
–

PhD candidate
Sam Edwards
Supervisors:
- Henrik de Fine Licht, Copenhagen University (DK)
- Bryony Williams, Exeter University (UK)
Outline
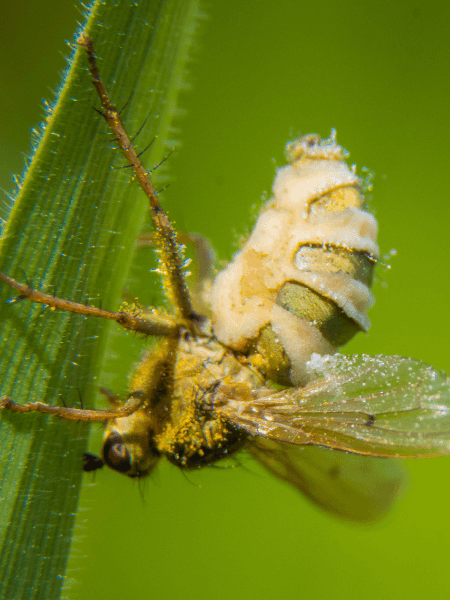
Some entomopathogenic parasites, notably fungi, can take over and manipulate the behaviour of infected insects for their own benefit, turning their hosts into “zombies”. These behavioural manipulations often increase fungal transmission to new susceptible hosts, and contribute to the epizootic capacity of the pathogen. This project aims to determine pathogen beneficial host manipulation by Entomophthora muscae in houseflies (Musca domestica) and by insect-infecting microsporidia. Additionally, houseflies are natural vectors of >100 different bacterial and viral food-borne human diseases, and like a number of other insect hosts suffering from microsporidia infection, are mass-reared in feed production, thus stressing the importance of understanding infection mechanisms.
During the final stages of a ~six-day infection from the exposure to infective E. muscae conidia, the obligate parasitic fungus manipulates its housefly host to perform a sequence of characteristic behaviours. The pathogenic fungus rings down the infection curtain by puppeteering its host in the following steps: 1) climb to an elevated position, 2) affix its proboscis to the substrate, 3) bow with raised wings and finally 4) die. This sequential performance is the curtain call of the infection. Following death, the fungus then erupts through the intersegmental membrane of the abdomen to sporulate (see photo 1).
The mechanisms involved in the fungal puppeteer’s manipulation of houseflies are unknown. To uncover these mechanisms, I will characterise and collect detailed information on the behaviour alterations visible in the insect host. Having characterised the intricacies of the manipulated behaviours, the aim now is to align what is occurring at a molecular level to allow for these manipulations. At the University of Copenhagen, I have optimised a RNA extraction protocol for extracting high-quality total RNA from fly heads to uncover the genes and molecules secreted by E. muscae during the conversion of these manipulations. I will use dual RNAseq on infected fly heads to discover what is expressed in the brain during manipulation.
This optimisation of RNA extraction from fungus-infected insects will also form the foundation for the second major goal of this project, based at the University of Exeter, involving microsporidia modification of host metabolites and homeostatic levels. Ultimately, I want the project to culminate in identifying co-evolutionary mechanisms of infection by my two pathogens.
Project 3
Interactions between pathogens infecting crickets:
who paves the way for the other?
Pathogens, especially the Acheta domesticus densovirus (AdDV), are threatening the mass production of house crickets Acheta domesticus. This pathogen is present in almost every cricket stock in a covert state. When it becomes virulent, AdDV eradicates the populations rapidly. I will examine the role of co-infections with bacterial and fungal entomopathogens and other stressors as possible triggers for the virulence of AdDV.

PhD candidate
Jozsef Takacs
Supervisors
- Annette Bruun Jensen, Univeersity of Copenhagen (DK)
- Vera Ros and Joop van Loon, Wageningen University (NL)

Outline
Insects have great potential to make our food chains circular. The house cricket Acheta domesticus is a promising candidate for food and feed purposes, but viral pathogens can cause epizootics in cricket rearings. The A. domesticus densovirus (AdDV) is the main threat, which already caused serious losses in commercial cricket production. AdDV is present in most known A. domesticus stocks, mostly in a covert state. During my research, I am exposing crickets to bacterial (Serratia marcescens) and fungal (Metarhizium brunneum) entomopathogens to see if these could trigger the AdDV virus outbreaks.
The aims of the project are:
- Compare the effects of co-infections (bacterium-fungus, virus-fungus and bacterium-virus) on cricket mortality, growth, developmental time and behaviour
- Evaluate synergistic versus antagonistic effects of pathogens on cricket fitness
- Determine if and how vertical virus transmission occurs
I started with the culturing and exposure system for the selected fungal and bacterial pathogens and test those as single pathogen infections in the crickets. This will allow to determine non-lethal dosages, as the aim is to stress the individuals to a point where AdDV becomes active. I have also confirmed AdDV presence in the cricket stock used, by screening different body parts, hemolymph and frass of the crickets. Throughout the project, I will focus on collecting quantitative data, by using and developing RT qPCR methods for each of the pathogens. This will be done preferably using either cricket frass or hemolymph, allowing for a non-destructive sampling method during the co-infection experiments. Different immune assays, such as PO activity and lysosome-like activity will be used to measure the status of the immune systems during the infections. Based on the data collected, I will set up a model on the co-infections, aiming to gain insight into development of disease outbreaks. In the last phase of the project, virus transmission will be examined, where I will determine if and how vertical transmission of the virus occurs.
Project 4
Microsporidia infection and virulence at varying population densities
Little is known about what future risk Microsporidia pose to insects reared for food and feed. However, past experiences with intensive invertebrate rearing indicate that these parasites, whilst generally benign, can rapidly sweep through populations causing extensive damage.
My work aims to assess the diversity of microsporidia that may affect reared insect and to study the effect of insect host population densities on the transmission and virulence of these parasites. This project takes place at the University of Exeter and the University of Copenhagen.
My research project entitled “Microsporidia infection and virulence at varying population densities” is well fitted in this WP as I will study interaction between microsporidia and their hosts under certain stressors (i.e. host density, and potentially co-infection).

The Living Systems Institute
University of Exeter.

Outline
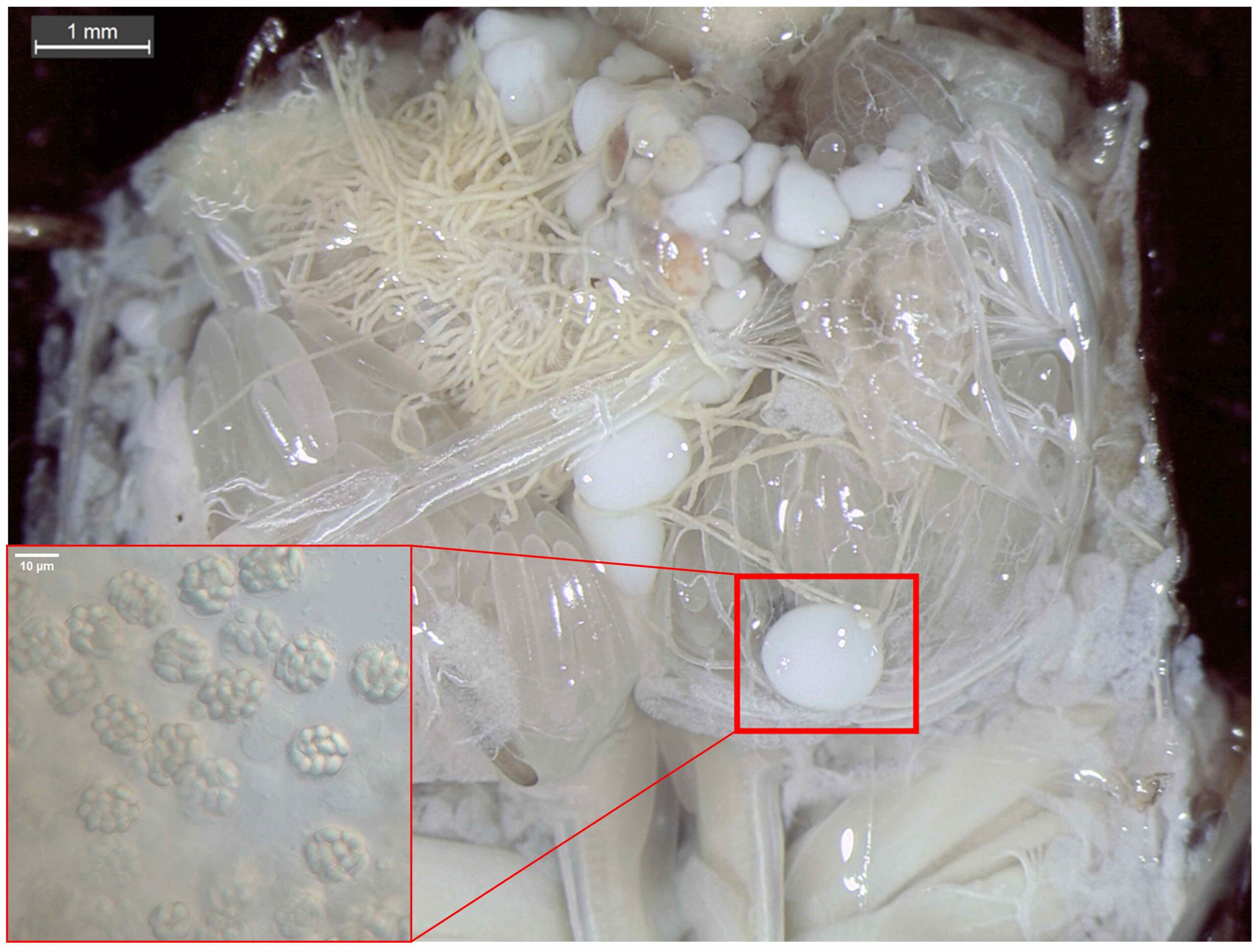
Microsporidia are a group of obligate intracellular parasites that infect a huge range of different animals. They often cause benign infections and go unnoticed. However, when hosts become immunocompromised or stressed these pathogens can cause severe disease, called microsporidiosis. This has been the case with microsporidia infections in honeybees that are affected by other stressors such as pesticides and other microbes, and in shrimp farms where animal are reared in high density, high stress conditions.
Since the risk of microsporidia to the rearing industry is not fully assessed, I will start to carry out a broad survey of parasites in samples from pet shops and other industry partners. The focus will be on the insect models Galleria mellonella and Tribolium confusum, but I will expand the investigation to other key insect species such as Tenebrio molitor, Blaptica dubia, Shistocerca gregaria, Zophobas Morio, Locusta migratoria, Acheta domesticus and Hermetia illucens. In the survey, I will also look for other eukaryotic parasites such as gregarines, nematodes, plasmodium and amoeba species that also may represent future challenges to the insect rearing industry.
Two significant strategies for the detection of parasites will be used: (1) lab techniques involving microscopy observation and PCR of insects obtained from commercial rearing facilities, (2) screening of the NCBI Sequence Read Archives (SRA) database, using bioinformatics tools, where a lot of insect genomic and transcriptomic data are available but not analyzed for presence of microsporidia, which may have been incidentally sequenced.
Once we identify our insect hosts – parasites models, we will set up healthy and infected colonies in order to start bioassays. Before all bioassays, the pathogenicity of the parasites within our insect models will be confirmed with the Koch’s postulates and the kinetics of the infection determined under standard rearing conditions.
These bioassays, with experimental infections, will allow us to test the hypothesis that high host population densities can favor both increased transmission and virulence of pathogens. The transmission is measured as the rate at which susceptible hosts become infected individuals. The virulence of a parasite is defined as the host’s loss of fitness (longevity and fecundity). In addition, I will further look into the relationship between the host density and the host immunity under a parasite infection.
The aim of this project is to produce empirical data on microsporidiosis, and other diseases potentially triggered by other eukaryotic parasites, with changing insect density. I am convinced that this research project will help insect farmers and industries to better understand the risk of microsporidia under mass rearing condition, which is frequently overlooked, but can result in the collapse of an entire insect colony. This project will also bring new insights into other understudied eukaryotic parasites within reared insect species.
Project 5
Impact of multiple biotic and abiotic stressors on pathogenesis in mealworms and wax moths
I am interested in how biotic and abiotic stressors affect arthropod cultures for food and feed. It is known that climate change provokes physiological responses in insects, which in turn affect population growth. Coupled to this, is the threat of emerging infectious diseases, which can severely affect mass-reared populations of invertebrates where high densities facilitate disease transmission.

Outline
I will examine the impact of multiple stressors (temperature, CO2 and pathogens) using in vivo model insect mass culture systems. Primarily, my experiments will focus on Coleopteran larvae of the yellow mealworm Tenebrio molitor, which is mass-reared for food and feed. Later on, I will extend my project and challenge some of the insights gained in this system to examine comparable issues in the insect order Lepidoptera, such as the wax moth Galleria mellonella. Potentially I will also include an established model of aquatic arthropod system using amphipod spp. as a proxy for shrimp and crayfish arthropod cultures used for food and feed.
With my research, I seek to understand which challenges arise in mass-reared arthropod cultures in a changing climate. Moreover, I am investigating how the production of arthropods for food and feed can be optimised and the risk of disease outbreaks can be minimised at the same time.
My experiments will be completed in a tiered approach, adding different stressors to the system over time. To quantify the immune response in stressed insects I am measuring phenoloxidase activity and concentration of hemocytes in the hemolymph. Moreover, I am using qPCR to quantify the development of pathogens inside hosts during sublethal infections. To measure host fitness responses I am mainly looking at survival, growth and development time of challenged hosts. I will use mixed effects models to analyze my data of individual host fitness. Furthermore, I will deliver a model that shows the impact of combined stressors at the population level to provide recommendations to industry of best culture practice and key points of intervention to reduce disease outbreak risks.
I am employed at UKCEH (UK Centre for Ecology & Hydrology) and I am enrolled at the University of Leeds and the University of Copenhagen. During my PhD, I will spend time at all three institutions, which is a great opportunity for me to get to know different people working in related fields. Furthermore, I will get insight in different working environments and I can benefit from different resources available at these locations.
